Impax (Q4262)
Impax (Q4262)
Dual Layer Impax Membrane
Dual Layer Impax Membrane is a sterile allograft designed for optimal wound covering and protection during the treatment of wounds.
Dual Layer Impax Membrane Key Features & Properties:
- Provides a reliable protective wound covering backed by decades of science
- Dehydrated extracellular matrix acts as a scaffold supporting the native tissue
- Adheres easily to wounds including those with irregular surfaces
- 5-year shelf life at ambient temperature storage
Dual Layer Impax Membrane is an amniotic membrane allograft derived from a prescreened mother with a planned delivery. Dual Layer Impax Membrane is manufactured in compliance with FDA regulations and AATB guidance. The membrane is minimally processed to preserve the native structure of the tissue, dehydrated, and terminally sterilized. Dual Layer Impax Membrane is confirmed by the FDA Tissue Reference Group to meet the criteria for regulation solely under Section 361 of the PHS Act as defined in 21 CFR Part 1271

Impax Case Study 1
Case presented by Jacob Fassman, DPM; Podiatrist; Colorado Foot and Ankle; Colorado Springs, CO
A 74 YEAR OLD MALE with a history of diabetes, GERD, COPD, hypertension, atrial fibrillation, and hypercholesterolemia presented for an urgent visit due to left lower extremity ulcerations. The patient has a history of venous insufficiency and venous ulcerations.
- Location: Left leg
Wound type: Venous ulcer
Weeks to resolution: 4
Total number of Impax applications: 3

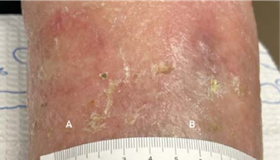
(A) Wound resolved
(B) 1.5 x 1 x 0.1 cm
Volume reduced: 70%
WEEK 3 Patient is improving; a third 4 x 4 cm Impax is applied, after which wounds are completely healed at fourth week follow-up
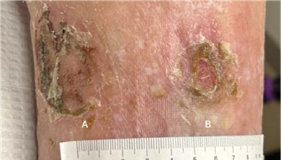
(A) 3 x 1.8 x 0.1 cm
Volume reduced: 80%
(B) 2.5 x 2 x 0.1 cm
Volume reduced: 33.3%
WEEK 2 Patient tolerating Impax well; a second 4 x 8 cm Impax is applied
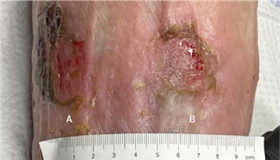
(A) 6 x 4.5 x 0.1 cm
(B) 2.5 x 3 x 0.1 cm
WEEK 1 After sharp excisional debridement, application of4 x 8 cm Impax.
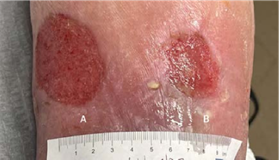
(A) 6 x 4.5 x 0.1 cm,
(B) 2.5 x 3 x 0.1 cm
INITIAL VISIT Incision, draining, and debridement, with Impax to be applied at next follow-up
Impax Case Study 2
Case presented by Jacob Fassman, DPM; Podiatrist; Colorado Foot and Ankle; Colorado Springs, CO
A 70 YEAR OLD FEMALE with osteoarthritis, hypertension, and neuropathy presented with three pressure/neuropathic ulcers on right foot that had been present for four months with a history of infection. Treated at wound clinic without significant improvement. Referred to vascular specialist who determined adequate blood flow for healing and no significant PAD.
- Location: Right foot
Wound type: Pressure/neuropathic ulcer
Weeks to resolution: 13
Total number of Impax applications: 6
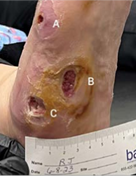
(A) 0.9 x 0.8 x 0.3 cm
(B) 4.6 x 3.3 x 0.2 cm
(C) 2 x 1.2 x 0.3 cm
INITIAL VISIT Post-debridement applied 2 x 2 cm Impax to all wounds
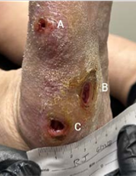
(A) 0.2 x 0.3 x 0.2 cm
Volume reduced: 94%
(B) 1.1 x 0.5 x 0.2 cm
Volume reduced: 96%
(C) 0.6 x 0.8 x 0.3 cm
Volume reduced: 80%
2-WEEK VISIT Post-debridement applied 2 x 2 cm Impax to all wounds
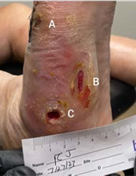
(A) Wound resolved
(B) 1.8 x 0.4 x 0.1 cm
Volume reduced: 35%
(C) 0.3 x 0.6 x 0.3 cm
Volume reduced: 63%
6-WEEK VISIT After surgery to remove infected bone and resolution of infection; post-debridement applied 2 x 2 cm Impax to all wounds
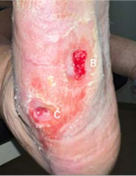
(B) 1.1 x 0.4 x 0.1 cm
Volume reduced: 39%
(C) 0.3 x 0.4 x 0.2 cm
Volume reduced: 56%
8-WEEK VISIT Post-debridement applied 2 x 2 cm Impax to all wounds
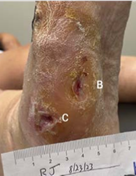
(B) 0.5 x 0.1 x 0.1 cm
Volume reduced: 89%
(C) 0.6 x 1 x 0.2 cm
Volume increased: 400%
10-WEEK VISIT Post-debridement applied 2 x 2 cm Impax to all wounds

(B) Wound resolved
(C) 0.4 x 0.6 x 0.3 cm
Volume reduced: 40%
12-WEEK VISIT Post-debridement applied 2 x 2 cm Impax to wound; all wounds resolved at week 13


